Details of any medical device’s manufacturing processes must be completely traceable so manufacturers can monitor product quality, make timely updates, and quickly recall defective items. Traceability extends from the original equipment manufacturer (OEM) to suppliers of individual components.
Should defects occur, cradle-to-grave tracking makes it possible to pinpoint exactly where changes in product quality initiated.
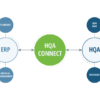
High QA’s software keeps measurement and inspection of medical parts – from all suppliers – on the same page.
”Quality information management software gets all medical device manufacturing process contributors on the same page for part dimensions, tolerances, and data reporting – maximizing process efficiency.